Albertsons ‘Non-Drowsy’ Signature Care Cold and Flu Medicines Cause Drowsiness, Class Action Claims
by Erin Shaak
Gibson v. Albertsons Companies, Inc.
Filed: February 4, 2022 ◆§ 1:22-cv-00642
Signature Care-brand “non-drowsy” cold and flu medicines sold by Albertsons contain an ingredient known to cause drowsiness, a class action alleges.
Illinois
Signature Care-brand “non-drowsy” cold and flu medicines sold by Albertsons Companies, Inc. are misleadingly labeled in that they contain an ingredient known to cause drowsiness, a proposed class action alleges.
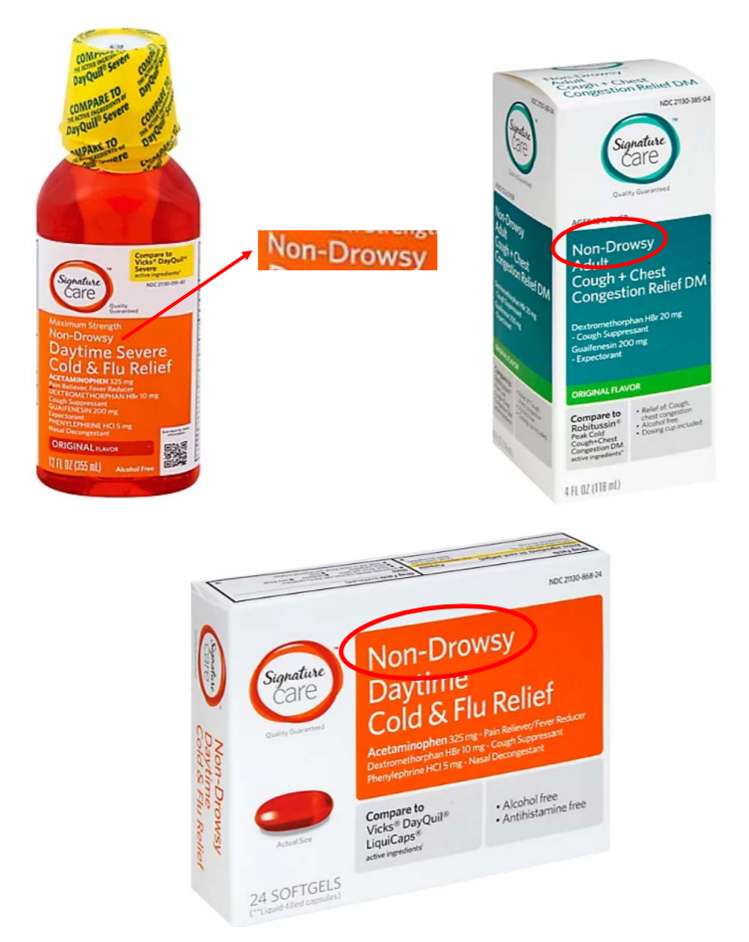
The 26-page lawsuit claims the grocery store chain’s private-label generic versions of brands like DayQuil and Robitussin are falsely advertised as non-drowsy, daytime-use medicines even though they contain the active ingredient dextromethorphan hydrobromide (DXM), a common side effect of which is drowsiness. According to the suit, Albertsons’ labeling of the over-the-counter cold and flu medicines as “Non-Drowsy” and “Daytime” misleads consumers into believing that the products will not cause drowsiness, or that drowsiness is not a side effect of taking the medication.
“In reality,” the complaint alleges, “the Products cause drowsiness, which in effect destroys the primary reason for purchasing the ‘Daytime’ Products in the first place – for use during the day, when consumers do not want to be drowsy.”
The lawsuit argues that consumers would not have purchased the Signature Care cold and flu medicines, or would have paid less, had they known the products could cause drowsiness.
According to the case, Albertsons sells Signature Care-brand “Non-Drowsy” medications as an alternative to its “Nighttime” cold and flu relief products, and consumers purchase these “Daytime” medicines for the specific purpose of avoiding drowsiness, the suit says.
The non-drowsy medications, however, do not disclose anywhere on their labels that they may cause drowsiness, or that a common side effect is drowsiness, the lawsuit alleges. Per the case, drowsiness is a documented side effect of dextromethorphan hydrobromide (DXM), even at the recommended dosages.
The lawsuit claims the defendant’s labeling of the products is false and misleading and violates state consumer protection statutes and the Federal Food, Drug, and Cosmetics Act.
The case looks to represent anyone who purchased a Signature Care product labeled as “non-drowsy” that contained DXM in the U.S. during the applicable statute of limitations period.
Get class action lawsuit news sent to your inbox – sign up for ClassAction.org’s newsletter here.
Video Game Addiction Lawsuits
If your child suffers from video game addiction — including Fortnite addiction or Roblox addiction — you may be able to take legal action. Gamers 18 to 22 may also qualify.
Learn more:Video Game Addiction Lawsuit
Depo-Provera Lawsuits
Anyone who received Depo-Provera or Depo-Provera SubQ injections and has been diagnosed with meningioma, a type of brain tumor, may be able to take legal action.
Read more: Depo-Provera Lawsuit
How Do I Join a Class Action Lawsuit?
Did you know there's usually nothing you need to do to join, sign up for, or add your name to new class action lawsuits when they're initially filed?
Read more here: How Do I Join a Class Action Lawsuit?
Stay Current
Sign Up For
Our Newsletter
New cases and investigations, settlement deadlines, and news straight to your inbox.
Before commenting, please review our comment policy.